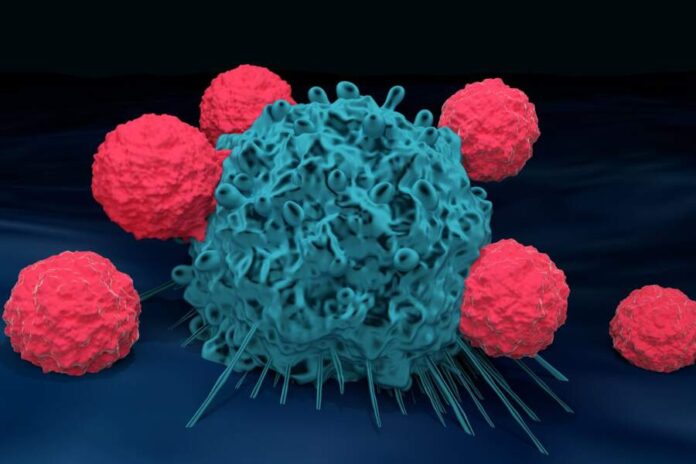
CAR-T therapy effectively trains the body’s natural T cells to target and attack cancer, giving patients a much better chance at positive treatment outcomes. This immunotherapy approach is showing great results, too, proving up to 90% effective in treating certain types of blood cancer. In some cases, patients even go into long-term remission after completing their CAR-T therapy treatments.
So, what’s the biggest thing holding this treatment back from becoming a primary therapy? A relatively high risk of serious side effects. Fortunately, Israeli and Australian researchers have been working together to overcome that barrier once and for all. Through their joint efforts, they aim to reduce the risk of toxic side effects and greatly improve the safety of CAR-T cell therapy.
Common CAR-T Cell Therapy Side Effects
In past studies, around 50% of patients given CAR-T therapy went on to develop many complications, including:
- Fever
- Respiratory distress
- High blood pressure
Ideally, new medical therapies should show a reasonable balance between the efficacy of the approach and its overall safety. With such a high occurrence of side effects – and some of them being quite serious – CAR-T cell therapy doesn’t yet offer that ideal balance.
For that reason, this treatment is only acceptable to use after exhausting all the other treatment options. By using CAR-T therapy as a last resort treatment, it’s not able to act on the cancer cells as quickly, potentially limiting its overall efficacy in the end.
Why CAR-T Cell Therapy Causes a Toxic Response
When patients undergo CAR-T therapy, they must first have their T cells extracted and sent to the lab. While at the laboratory, medical experts re-engineer the cells to produce proteins called chimeric antigen receptors (CARs) all over their surface. These high-tech proteins serve as an artificial sensor, allowing them to target cancer cell proteins with laser precision.
After upgrading the T cells, they’re given time to multiply before getting injected back into the patient’s bloodstream. As soon as the T cells start circulating through the patient’s body, they get to work in tracking down and attacking the cancer cells. If the patient’s tumor burden is high enough, a toxic response occurs as their body tries to cope with the battle waging within.
The overall strength of the toxic response is not easy to predict either. So, patients never truly know if they’ll experience serious side effects until the T cells enter their system and get to work. At that point, it’s impossible to stop the supercharged CAR-T cell response, leaving patients dealing with whatever side effects come their way.
New Study Looks into Balancing Efficacy and Safety
To overcome this difficult problem, researchers at the Weizmann Institute of Science and the Walter and Eliza Hall Institute of Medical Research (WEHI) have teamed up. Through their joint study, they set out to find that perfect balance of efficacy and safety for CAR-T therapy patients.
In previous studies, researchers tried to finetune the CAR-T cell by modifying the ends of the artificial sensor. Since the ends either target or attack the cancer cells, the modifications did little to reduce side effects.
So, instead of working from either end, researchers at WEHI and the Weizmann Institute of Science have set their sights on the middle section. Their findings published in the eLife journal revealed that modification of that center section allowed them to create CARs with varying potency levels. With that move, it’s possible to adjust the efficacy of the treatment enough to kill the cancer cells without causing a toxic response.
Although their research seems promising, they still have to test that their findings work in a clinical setting. So, in the next stage of the process, they’ll bring in volunteers to try the newfound approach to CAR-T therapy as a first line of treatment instead of the last resort.